Introduction
The treatment of Erdheim-Chester disease (ECD) has been revolutionized by the discovery of MAPK/ERK pathway mutations, most common being BRAF-V600E (50%). The US-FDA approved single-agent vemurafenib for BRAF-V600-mutated ECD in 2017 based on a phase II trial that included 22 patients with ECD and 4 with Langerhans cell histiocytosis (LCH), resulting in 100% overall response rate (ORR) by FDG-PET criteria (Diamond et al. JAMA Onc 2018). However, adverse events (AEs) to vemurafenib led to treatment interruption and/or dose modification in all patients, and drug discontinuation in 8 (31%) patients. Long-term outcomes and tolerability of BRAF-inhibitor (BRAFi) monotherapy in real world setting are unknown. In this study, we aimed to address these questions utilizing a large cohort of patients with ECD.
Methods
Registry-based ( NCT03329274) study of patients with ECD who received BRAFi (vemurafenib or dabrafenib) as any line of treatment. The registry captures detailed longitudinal clinical, treatment, and outcomes data through medical record abstraction. For this study, we focused on the events of discontinuation, dose reductions, and AEs to BRAFi therapy. Time-to-event analyses were conducted using Kaplan-Meier method from the date of initiation of BRAFi. Primary endpoint of the study was event-free survival (EFS), defined as time to progression of disease, discontinuation or alteration of therapy due to any cause (including addition of a MEK inhibitor), or death. Secondary endpoints included progression-free survival (PFS), defined as time to progression of disease or death, and overall survival (OS).
Results
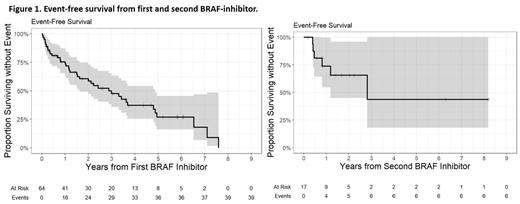
We included 64 patients with ECD (including 7 with mixed ECD/LCH) in this study. Median age at diagnosis was 56y (range, 26-77) and at initiation of BRAFi was 57y (range, 26-77). Of these, 59 (92%) were BRAF-V600E, 2 (3.1%) were BRAF-wildtype, 1 had BRAF-V471F, 1 had BRAF-PICALM fusion, and 1 had unknown BRAF status. The distribution of organ involvement was bone (91%), neurologic (78%), brain parenchyma (62%), cardiovascular (58%), retroperitoneum (58%), skin (25%), and pulmonary (19%). BRAFi therapy was used as first-line in 38 (59.3%) and as subsequent line in the rest (median 1, range 1-7). The most common initial BRAFi used was vemurafenib (80%) followed by dabrafenib (20%). The median starting dose of vemurafenib was 720 mg bid (range 480-960) and end dose was 480mg bid (range, 240-480). The median start dose of dabrafenib was 100mg bid (range 50-150) and end dose was 50mg bid (range 50-100). Initial dose reduction of BRAFi was required due to AEs in 28 of 52 cases (54%) with final dose data available, encompassing cutaneous (43%), generalized symptoms or fatigue (18%), >1 system affected (14%), lab abnormalities (11%), musculoskeletal (7%), and other (7%) toxicities. Of 60 patients with response data, a response was seen in 51 patients or 85% (42 partial response, 9 complete response, 8 stable disease, and 1 progressive disease). The median follow-up duration was 3.9 years (range 0.2-8.8). Single-agent BRAFi therapy was discontinued in 38 (59%) patients who received it initially (vemurafenib 63% vs. dabrafenib 46%, p=0.28). Reasons for discontinuation included AEs (n=20, 53%), addition of MEK-inhibitor (18%) for reducing cutaneous toxicities (n=5) or augment CNS response (n=2), disease progression (n=4, 11%), death (n=3, 8%), or others (n=4, 11%). 17 patients were rechallenged with a BRAFi after initial discontinuation, and 6 (35%) discontinued it again. The median EFS for the entire cohort from first BRAFi treatment was 2.99y (95% CI 1.6-4.8) and from second BRAFi was 2.82y (95% CI 1.19- not reached). The median PFS and OS were not reached, and 4 patients died at last follow-up (3 from ECD).
Conclusions
Almost 5 years after the FDA approval of vemurafenib for ECD, our real-world study from long-term follow-up of a large cohort of patients highlights high response rates, but at the cost of adverse events leading to dose reductions and treatment discontinuation in a large subset. Our results also highlight the entity of disease progression on BRAFi therapy. Our results underscore the importance of developing therapies that are efficacious and well-tolerated in this incurable disease.
OffLabel Disclosure:
Goyal:Opna Bio: Membership on an entity's Board of Directors or advisory committees. Lacouture:Roche: Consultancy; OnQuality: Consultancy; Lutris: Consultancy; Seattle Genetics: Consultancy; Loxo: Consultancy; Genentech: Consultancy; Trifecta: Consultancy; RBC/La Roche Posay: Consultancy; Kintara: Consultancy; Deciphera: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Novocure: Consultancy; Johnson and Johnson: Consultancy, Research Funding; AstraZeneca: Research Funding; Oncoderm: Consultancy; Apricity: Consultancy. Rampal:Zentalis: Research Funding; Stemline: Research Funding; Servier: Consultancy; Kartos: Consultancy; Morphosys/Constellation: Consultancy; Sumitomo: Consultancy; Incyte: Research Funding; Zentalis: Consultancy; Dainippon: Consultancy; Ryvu: Research Funding; Constellation: Research Funding; Karyopharm: Consultancy; Pharmaessentia: Consultancy; Galecto: Consultancy; CTI BioPharma Corp: Consultancy; Celgene-BMS: Consultancy; GSK-Sierra: Consultancy; Incyte: Consultancy. Diamond:Pfizer: Other: Unpaid editorial support; Opna Bio: Membership on an entity's Board of Directors or advisory committees; Springworks: Membership on an entity's Board of Directors or advisory committees; Day One Biotherapeutics: Membership on an entity's Board of Directors or advisory committees.
Dabrafenib for Erdheim-Chester disease.